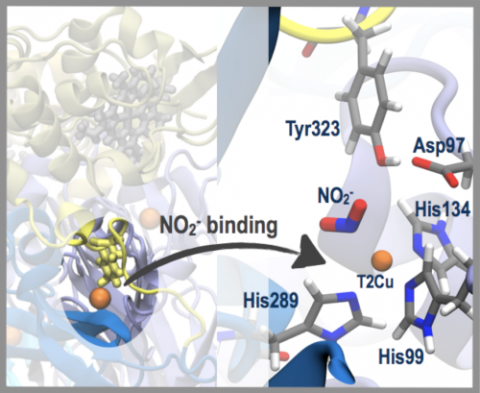
Copper-containing nitrite reductase enzymes (CuNiRs) play a key role in the global nitrogen cycle by reducing nitrite (NO2−) to nitric oxide (NO). CuNiRs come in two-domain and three-domain forms, where the former have one cupredoxin domain and the latter have an additional cupredoxin or haem c domain. In two-domain CuNiRs, nitrite binding at the “T2Cu” active site is observed crystallographically, but in three-domain RpNiR it is experimentally elusive. It is hypothesized that a tyrosine residue from the linker part of the haem c domain blocks the substrate access channel and impedes nitrite binding.
With no experimental structures of substrate binding available we have used ChemShell QM/MM calculations to predict nitrite binding to native RpNiR. Our calculations suggest that NO2− binds to the T2Cu site in its oxidized Cu(II) state, but this occurs in an asymmetric L-shaped orientation via the nitrogen atom instead of the “top-hat” symmetric bidentate binding via the oxygen atoms observed in the corresponding two-domain CuNiRs. This observation is corroborated by a recent crystal structure of nitrite bound to mutant RpNiR.
K. Sen, M. A. Hough, R. W. Strange, C. W. Yong, and T. W. Keal, “A QM/MM Study of Nitrite Binding Modes in a Three-Domain Heme-Cu Nitrite Reductase“, Molecules, 2018, 23, 2997.
